Decipher® Prostate Metastatic
Test Report Overview
Gain confidence in interpreting Decipher Prostate test results for your patients with metastatic prostate cancer. The below tutorial walks you through each section of the report, highlighting the Decipher Score, risk group and clinical implications, to support informed treatment planning.

Page 1: contains the Decipher Score, risk estimates, and an interpretation of the score

1. Decipher Risk & Interpretation
- For a metastatic patient, the score is classified as:
- Decipher Higher Risk (>0.85)
- Decipher Lower Risk (≤0.85)
- Cut-point (0.85) reflects the median Decipher score across 1,221 retrospectively and prospectively tested mCSPC patients which ranged from 0.80-0.90
- Report interpretation is tailored to the patient’s clinical presentation
(mCSPC – metastatic castration sensitive prostate cancer)
2. Risk Estimates
- Specific to the patient’s Decipher score and calculated using data from phase 3 clinical trials
- Reflect outcomes after treatment with ADT alone
- Likelihood of remaining non-castration resistant at 18 months
- Likelihood of being alive at 3 yrs
- Higher percentages indicate more favorable outcomes
- Visually highlighted based on the patient’s Decipher Risk (Higher or Lower) and disease volume (high or low)
Page 2: contains information on absolute benefit and clinical finding relevant to the patient
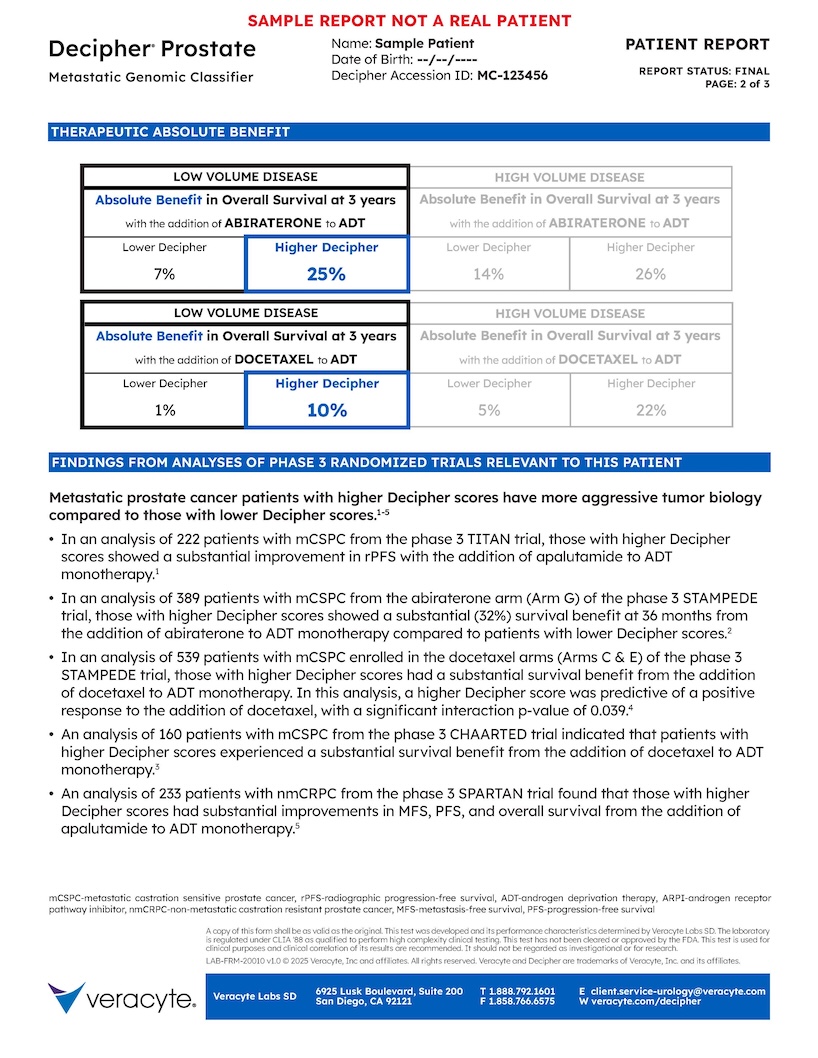
3. Absolute Benefit
- Refers to absolute overall survival benefit at 3 years from:
- Adding abiraterone to ADT (determined by data from STAMPEDE Arm G)
- Adding docetaxel to ADT (determined by data from CHAARTED and STAMPEDE Arm C)
- Absolute benefit values are derived from the published trial data and are fixed
- Visually highlighted based on the patients Decipher risk (Higher or Lower) and disease volume (high or low)
4. Clinical findings
- Relevant findings from post-hoc analyses of phase 3 randomized trials
- Content is based on the patient’s Decipher risk (Higher or Lower)
Page 3: contains the test description, intended use information, confidence intervals, and references

5. Test Description
- How the test is performed
- Definitions for each of the endpoints reported
- Provides detail on the cut-points for Decipher risk groups
6. Intended Use
- Decipher Prostate Metastatic is intended for use in patients with metastatic prostatic adenocarcinoma. The test is performed on the most recent biopsy or radical prostatectomy specimen prior to treatment with radiation or hormone therapy
7. Confidence Intervals
- The 95% confidence intervals for each endpoint reported
8. References
- For each of the clinical studies cited in the report