Personalized Insights for Bladder Cancer Care
Veracyte’s Decipher Bladder reveals the molecular subtype of a patient’s bladder cancer, providing actionable insights that can help you make informed, personalized treatment plans and ensure the best care possible for your patients.


What is Decipher Bladder?

A transcriptomic subtyping tool that provides a deep biological characterization of your patients’ bladder cancer derived from 219 genes

Intended for patients with high-grade T1 through T4a bladder cancer

Performed on tumor tissue collected during a trans urethral resection of a bladder tumor (TURBT)
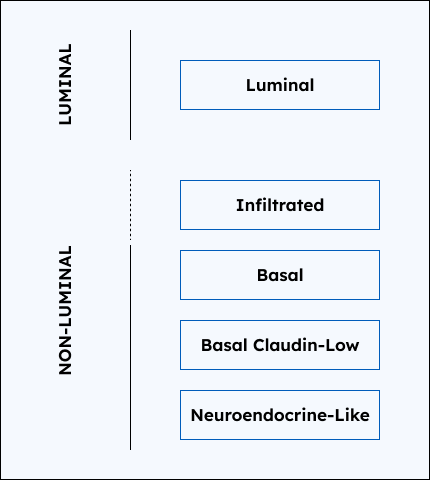
Decipher Bladder provides the tumor molecular subtype of your patient’s bladder cancer, which:
- May help determine a patient’s risk of upstaging at time of
radical cystectomy1 - May help identify patients who might benefit most from cisplatinbased neoadjuvant chemotherapy (NAC) prior to
radical cystectomy2 - Identifies patients with neuroendocrinelike tumors, which are associated with a poor prognosis and may benefit from rapid referral to medical oncology3
- May be useful in identifying optimal candidates for immune checkpoint inhibitor therapy4
Page 1 contains the Molecular Subtype and risk estimates

1. Molecular Subtype
- The molecular subtype in which the patient tumor has the highest probability of belonging
- Luminal tumors tend to be associated with less aggressive disease while non-luminal tend to be associated with more aggressive disease
2. Risk Estimates
- Summary based on the patient’s tumor molecular subtype and relevant clinical findings
- Includes the risk of upstaging the patient may have at RC and benefit from NAC prior to RC
Page 2 contains case analysis, interpretation, and clinical findings.

3. Subtype Probabilities
The probabilities of the patient tumor belonging to each of the 5 molecular subtypes
4. Clinical Findings
Clinical study results relevant to this patient
5. Test Description
An overview of how the test is performed
6. Intended Use
Decipher Bladder is intended for use in patients with AJCC Stage I to IIIA bladder cancer who are candidates for definitive therapy
7. References
References for each of the clinical studies cited in the report.
How to Order
Interested in Ordering
Decipher Testing?
Learn more on how to order the Decipher Bladder Genomic Classifier.
Resources
Access resources &
publications about Decipher
View resources and publications about Decipher testing.
Decipher Bladder Resources
Physician Bladder Brochure
Sample Decipher Bladder Test Report
Decipher Bladder Requisition
Decipher Financial Assist Form
Don’t see what you’re looking for?
Visit these pages for more information or leave us a message on the contact form.
Frequently Asked Questions
How can I place an order?
Physicians can place an order by filling out a requisition and submitting the required forms using fax, email, or the Decipher portal. Visit our How to Order page to learn more.
How can I set up a portal account to begin ordering?
Please fill out a contact form here and a representative will reach out to help set up a portal account.
How can I contact a local representative to learn more?
Please fill out a contact form here to contact a representative to learn more about Decipher testing. For questions related to billing and insurance, please reach out to Veracyte’s billing team at [email protected] or call us at 1.888.792.1601 (select option #3).
Is the Decipher Prostate Genomic Classifier or Decipher Bladder Genomic Classifier FDA cleared?
The Decipher Prostate Genomic Classifier and the Decipher Bladder Genomic Classifier are available as part of Veracyte’s Clinical Laboratory Improvement Amendments (CLIA)-validated laboratory developed test (LDT) service. FDA approval or clearance is not required for the Decipher Prostate Genomic Classifier or Decipher Bladder Genomic Classifier.
Veracyte Labs SD, which performs Decipher testing is accredited/licensed by the following:
- College of American Pathologists
- Centers for Medicare and Medicaid Services (CMS) CLIA
- California Department of Public Health
- New York State Department of Health
- Pennsylvania Department of Health
- State of Rhode Island and Providence Plantations Department of Health Center for Health Facilities Regulation
- Maryland Department of Health, Office of Health Care Quality
Can the Decipher Bladder Genomic Classifier be ordered if I live outside the United States?
Currently, Decipher testing is only available within the United States. Decipher testing is available as part of Veracyte’s CLIA-validated laboratory developed test (LDT) service. Veracyte’s exclusive global access to the nCounter Analysis System for diagnostic testing will allow us to make our genomic classifiers – currently performed in our centralized labs for patients in the U.S. – available to physicians and their patients globally. We plan to adapt Decipher genomic testing to the nCounter Analysis System in the next few years. Feel free to fill out our contact us form should you be interested in Decipher testing outside the United States.
References
- Lotan Y et al., Molecular Subtyping of Clinically Localized Urothelial Carcinoma Reveals Lower Rates of Pathological Upstaging at Radical Cystectomy Among Luminal Tumors. European Association of Urology, Vol. 76, Issue 2, 2019, Pages 200-206.
https://doi.org/10.1016/j.eururo.2019.04.036 - Lotan Y et al., Patients with Muscle-Invasive Bladder Cancer with Nonluminal Subtype Derive Greatest Benefit fromPlatinum Based Neoadjuvant Chemotherapy. The Journal of Urology, Vol. 207, 2021, Pages 541-550. www.auajournals.org/doi/10.1097/JU.0000000000002261.
- Grivas P et al., Validation of a neuroendocrine-like classifier confirms poor outcomes in patients with bladder cancer treated with cisplatin-based neoadjuvant chemotherapy. Urologic Oncology: Seminars and Original Investigations, Vol. 38, Issue 4, 2020, Pages 262-268.
https://doi.org/10.1016/j.urolonc.2019.11.004. - Necchi A et al., Molecular Subtyping and immune-gene signatures identify a subset of early bladder tumors as candidates for single-agent immune-checkpoint inhibition. Urologic Oncology: Seminars and Original Investigations, Vol. 39, Issue 10, 2021, Pages 734.e11-735.e17.
https://doi.org/10.1016/j.urolonc.2021.06.011.
This page is intended for US healthcare professionals only.