Decipher® Prostate: The Most Validated Genomic Test for Prostate Cancer
Decipher Prostate delivers trusted genomic insights to help physicians and patients make confident, personalized treatment decisions across the prostate cancer continuum. Validated in over 90 peer-reviewed studies and 200,000 patients, this tissue-based test is a highly accurate predictor of metastasis, prostate cancer-specific mortality, and overall survival.


Decipher is the Most Validated Prostate Cancer Gene Expression Test
200,000+
Prostate cancer patients included in published studies with Decipher Prostate
25+
Prospective randomized clinical trials that include Decipher Prostate
Incorporated as an integral biomarker in multiple National Cancer Institute-sponsored trials
90+
Publications demonstrating the clinical validity and utility of Decipher Prostate
22-Gene Genomic Classifier (GC) (Decipher) is the Only Gene Expression Test Included in V.1.2025 of the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer:
The only gene expression test included in V.1.2025 of the NCCN Guidelines® as part of the updated “Advanced Tools” table located in the Principles of Risk Stratification and Biomarkers section (PROS-H).
“It is recommended to use models that have robust validation, ideally with high-quality, long-term clinical trial data, which usually comes from randomized trials and across multiple clinical trials.”
“Only advanced tools with Simon level of evidence of I,1 or specific alterations linked to FDA-approved treatments are shown in Table 1.
- A comprehensive list of advanced tools that do not reach the threshold of level I evidence is outside the scope of this guideline, but examples of such tests include gene expression tests (ie, 31-gene assay [Prolaris] and 17-gene assay [Genomic Prostate Score]).”
PROS-H, 3 of 7
PRINCIPLES OF RISK STRATIFICATION AND BIOMARKERS*
Table 1. Advanced Tools
Localized
| Category | Tool | Predictive | Prognostic | Prognostic Endpoint Trained Fora | Treatment Implications | |
|---|---|---|---|---|---|---|
| Gene Expression | 22-Gene Genomic Classifier (GC) (Decipher) 2-4 | Not Determined | Yes | DM | See Table 2** | |
Post-RP
| Category | Tool | Predictive | Prognostic | Prognostic Endpoint Trained Fora | Treatment Implications | |
|---|---|---|---|---|---|---|
| Gene Expression | 22-Gene GC 5-6 | Not Determined | Yes | DM | See Table 2** | |
DM = Distant Metastasis
PROS-H, 4 of 7, Table 1
Note: All recommendations are category 2A unless otherwise indicated.***
***Please note that “category 2A” is derived from the National Comprehensive Cancer Network® (NCCN®) Categories of Evidence and Consensus (CAT-1), which is independent from the Simon levels of evidence1 used to warrant inclusion in Table 1 above. The category 2A footnote above applies to recommendations only, and means that, based upon lower-level evidence, there is uniform NCCN consensus (≥85% support of the Panel) that the intervention is appropriate. Simon level II evidence pertains to the nature and level of clinical trial evidence, and includes only levels IIB and IIC, as there is no Simon Level IIA.1
References
1. Simon RM et al. J Natl Cancer Inst 2009;101:1446-1452. 2. Spratt DE et al. Int J Radiat Oncol Biol Phys 2023;117:370-377. 3.Nguyen PL et al. Int J Radiat Oncol Biol Phys 2023;116:521-529. 4. Spratt DE et al. J Clin Oncol 2018;36:581-590. 5. Feng FY et al. al. JAMA Oncol 2021;7:544-552. 6. Dal Pra Aet al. Ann Oncol 2022;33:950-958.
*Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.1.2025. © 2024 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. NCCN = National Comprehensive Cancer Network® (NCCN®). **Table 2 is available in PROS-H, 5 of 7 in v1.2025 of the NCCN Guidelines for Prostate Cancer. a. The listed models and biomarkers may have demonstrated they are prognostic for additional endpoints. This column indicates what the original model or biomarker was trained for.
NCCN = National Comprehensive Cancer Network® (NCCN®).
Decipher Prostate is available in the United States as part of Veracyte’s CLIA-validated laboratory developed test (LDT) service, and FDA clearance is not required.
Localized Prostate Cancer
(Biopsy)
Localized Prostate Cancer
(Radical Prostatectomy)
Metastatic Prostate Cancer
(Biopsy or Radical Prostatectomy)
Decipher Prostate was Developed to Predict Metastasis
- Metastasis is the best surrogate endpoint for overall survival for prostate cancer.
- Discovery and validation studies were completed in tumor tissue from a high-risk cohort of localized prostate cancer patients.
20% of these patients developed distant metastases within 5 years. - Differential analysis of metastatic versus non-metastatic tumor RNA expression identified robust differences in the expression
of the 22 genes that comprise the Decipher Prostate Genomic Classifier.

639 Post-RP Patients
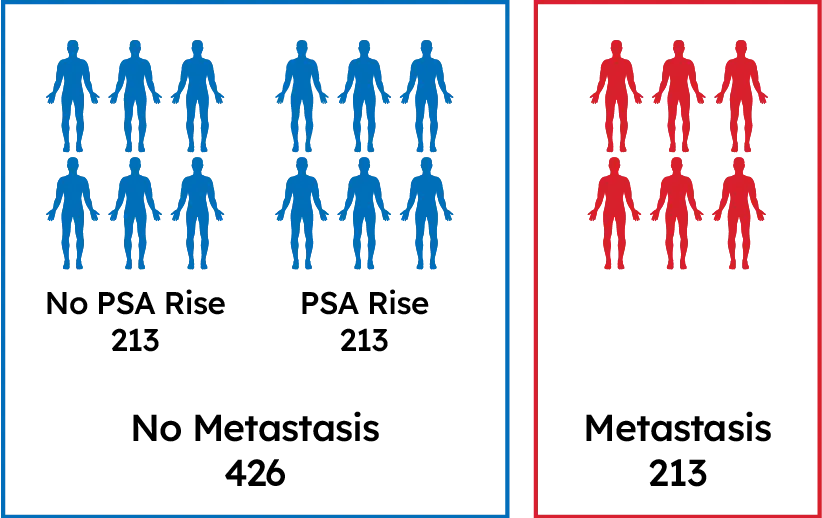
Patients with long term follow-up: Gleason 8-10 or Pre-Op PSA>20 ng/mL or Stage T3 or greater or SM+
Whole Transcriptome
Microarray
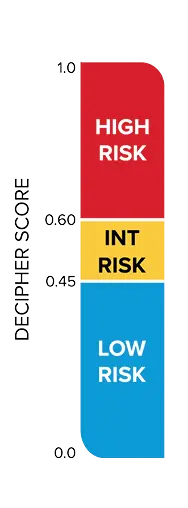
Decipher Risk
Well-annotated tumor registry with long-term treatment & outcomes data
Clinically high-risk patients with different 5-year outcomes: no PSA rise, PSA rise, & distant metastasis
Compared gene expression from tumor tissue of patients who developed metastasis vs. those who did not
Identified the 22 biomarkers that comprise the test

Decipher Prostate Resources
Decipher Prostate Requisition
Sample Test Reports
Don’t see what you’re looking for?
Visit these pages for more information or leave us a message on the contact form.
Frequently Asked Questions
How can I place an order?
Physicians can place an order by using the Decipher Physician Portal or by filling out a requisition and submitting the required forms via email or fax. Visit our How to Order page to learn more or speak with your local Decipher representative.
How can I set up a portal account to begin ordering?
For help with setting up a portal account, please fill out a contact form here or reach out to your local Decipher representative.
How can I contact a local representative to learn more?
Please fill out a contact form here and a representative will reach out to you
What is included in a patient’s Decipher Prostate test report?
Decipher Prostate reports (Biopsy, Radical Prostatectomy, or Metastatic) are designed to support risk stratification and clinical decision-making. The first page of each report provides the patient’s Decipher Score, corresponding genomic risk group (low, intermediate, or high for localized disease; lower or higher for metastatic disease), interpretation language, and risk estimates for prostate cancer-specific outcomes. The second page includes additional clinical context and detailed data specific to the individual patient.
Is the Decipher Prostate Genomic Classifier or Decipher Bladder Genomic Classifier FDA cleared?
The Decipher Prostate and Bladder tests are available as part of Veracyte’s Clinical Laboratory Improvement Amendments (CLIA)-validated laboratory developed test (LDT) service. FDA approval or clearance is not required for the Decipher Prostate or Bladder tests.
Veracyte Labs SD, which performs Decipher testing is accredited/licensed by the following:
- College of American Pathologists
- Centers for Medicare and Medicaid Services (CMS) CLIA
- California Department of Public Health
- New York State Department of Health
- Pennsylvania Department of Health
- State of Rhode Island and Providence Plantations Department of Health Center for Health Facilities Regulation
- Maryland Department of Health, Office of Health Care Quality
Can the Decipher Prostate Genomic Classifier or Decipher Bladder Genomic Classifier be ordered if I live outside the United States?
Currently, Decipher testing is only available to patients within the United States through Veracyte’s CLIA-validated laboratory service. We are actively exploring opportunities to expand access to Decipher testing internationally in the coming years. If you are located outside the U.S. and are interested in Decipher testing, please fill out our contact us form to let us know.
This page is intended for U.S. healthcare professionals only.
