Decipher Prostate Biopsy Test Report Overview
We want you to feel confident in every treatment decision you make using Decipher Prostate for your patients with prostate cancer, beginning with a clear understanding of how to interpret the key components of the biopsy test report.

Page 1 contains the Decipher Score, risk estimates, and an interpretation of the score.

1. Dynamic Report
The report is tailored to the clinical presentation of this patient
2. Decipher Score
- Reflects genomic risk of metastasis
- Determined by tumor biology alone, independent of clinical & pathological factors (e.g., Gleason, PSA)
- 22 genes, 7 biological pathways
- Continuous genomic risk score classified as low, intermediate or high
3. Interpretation
Summary based on this patient’s genomic risk & relevant clinical findings
4. Risk Estimates
- Calibrated to outcomes of patients treated with definitive therapy appropriate for this patient’s NCCN risk group
- Metastasis at 5 & 10 years
- Prostate Cancer Mortality at 15 years
- Adverse Pathology at RP (Grade Group 3-5, pT3b-T4, or lymph node involvement)
Page 2 contains case analysis, interpretation, and clinical findings.
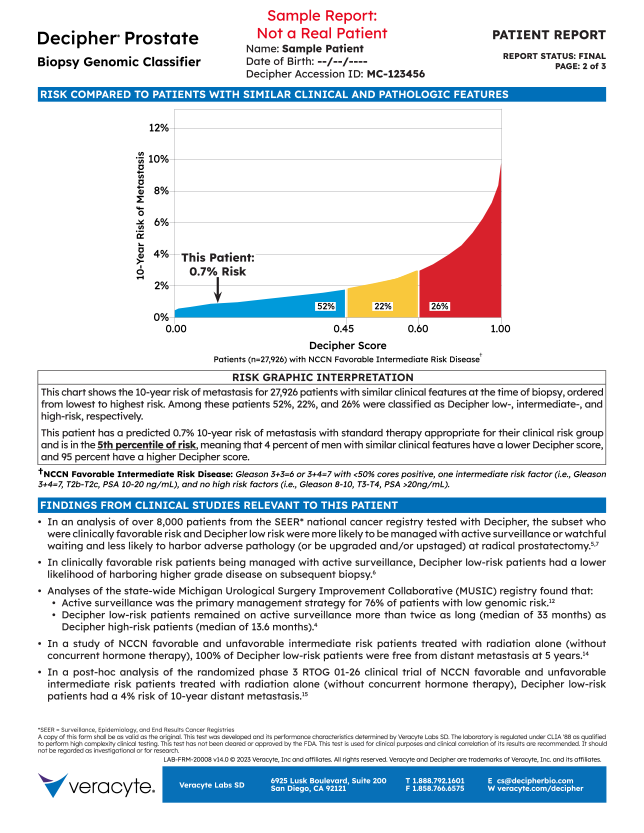
6. Interpretation
- Explains risk comparison graphic
- Provides this patient’s percentile rank in the tested population
5. Risk Comparisons
- This patient’s 10-year risk of metastasis with respect to 27,926 other NCCN Favorable Intermediate Risk patients
- The distribution of Decipher risk within the 27,926 patients is:
- 52% Decipher Low
- 22% Decipher Int.
- 26% Decipher High
7. Clinical Findings
Clinical study results relevant to this patient
Page 3 contains a test description, intended use info, confidence intervals, and references.

8. Test Description
- Description of:
- Decipher testing platform technology
- Risk estimates
- Cut-points separating Decipher low, intermediate & high
9. Intended Use
Decipher Prostate Biopsy is intended for use in biopsy specimens from patients with localized or pelvic node positive prostate cancer to help inform treatment decisions
10. Confidence Intervals
The 95% confidence intervals for each risk estimate (on page 1)
11. References
For each of the clinical studies cited in the report