Decipher Prostate is a tissue-based genomic test that provides actionable information to help physicians and their patients make treatment decisions across the spectrum of localized prostate cancer.


Decipher is the Most Validated Prostate Cancer Gene Expression Test
100,000+
Prostate cancer patients included in published studies with Decipher Prostate
25+
Phase 2 & 3 prospective randomized clinical trials that include Decipher Prostate
Incorporated as an integral biomarker in 7 National Cancer Institute-sponsored trials
80+
Decipher Prostate clinical validity &
utility publications
22-Gene Genomic Classifier (GC) (Decipher Prostate) is the Only Gene Expression Test with Simon Level 1B Evidence in the 2024 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)
“There are an extensive number of these tools created with substantial variability in quality of reporting and model design, endpoint selection, and quality and caliber of validation. It is recommended to use models that have high-quality and robust validation, ideally with high-quality, long-term clinical trial data, which usually comes from randomized trials and across multiple clinical trials.” PROS-H 1 of 8
| Risk Stratification: Selected Advanced Tools for Localized Prostate Cancer Post-Biopsy* | ||||||
|---|---|---|---|---|---|---|
| Category | Tool | Predictive | Prognostic | Prognostic Endpoint Trained Fora | Simon Level of Evidence1,b | Treatment Implications |
|
Gene Expression Testing |
22-gene genomic classifier (GC) (Decipher) | No | Yes | Metastasis | IB | See Table 3 |
| 31-cell cycle progression (CCP) Gene assay (Prolaris) | No | Yes | See footnotec | IIICd | ||
| 17-gene Genomic Prostate Score (GPS) assay | No | Yes | Adverse pathology | IIIC | ||
| Risk Stratification: Selected Advanced Tools Post-RP | ||||||
|---|---|---|---|---|---|---|
| Category | Tool | Predictive | Prognostic | Prognostic Endpoint Trained Fora | Simon Level of Evidence1,b | Treatment Implications |
|
Gene Expression Testing |
22-gene GC | No | Yes | Metastasis | IB | See Table 3 |
| 31-CCP gene assay | No | Yes | See footnotec | IVD | ||
| 17-gene assay | No | Yes | Adverse pathology | IVD | ||
PROS-H, 3 of 8
Table 2
- The listed models or variables may have demonstrated they are prognostic for additional endpoints. This column indicates what the original model was trained for.
- Simon level of evidence criteria are as follows1:
- 1A. Prospective clinical trial(s) designed to address tumor marker
- 1B. Prospective clinical trial(s) using archived samples with design that accommodates tumor marker utility, ≥1 validation study available with consistent results
- IIB. Prospective clinical trial(s) using archived samples with design that accommodates tumor marker utility, no validation studies available, or validation studies have inconsistent results
- IIC, Prospective observational registry, ≥2 validation studies available with consistent results
- IIIC, Prospective observational registry, no validation studies available, or 1 validation study with consistent or inconsistent results
- IVD, Small retrospective/observational studies with no prospective aspect
- IVD, Small retrospective/observational pilot studies with no prospective aspect, designed to determine biomarker marker levels in a population.
- CCP was not specifically trained for a clinical endpoint.
- The CCP biomarker is level IVD except for grade group 1 cancer where it is level IIIC, where CCP was independently associated with minor upgrading, but was not significantly associated with major upgrading or biochemical recurrence. Cooperberg MR, et al.Eur Urol 2021;79:141-149.
National Comprehensive Cancer Network® (NCCN®)
“Given the moderate prognostic performance of NCCN risk groups to risk stratify localized prostate cancer, there is intrinsic heterogeneity in prognosis within a given NCCN risk group. Thus, treatment recommendations for adjacent risk groups may be appropriate when using more accurate risk stratification methods in addition to NCCN risk group assignments.” PROS-H 1 of 8
| Treatment Implications for Advanced Tools: 22-Gene Genomic Classifier (GC) Assay* | |||
|---|---|---|---|
| Population | Score | Treatment Decision | Treatment Implications |
| NCCN Low-Risk | ≥0.6 | Active Surveillance Intensity Therapy vs. Radical Therapy |
Evidence: In a prospective multicenter statewide registry, GC high risk (≥0.6) was associated with shorter time on active surveillance and shorter time to treatment failure (TTF) for those who underwent radical therapy.2 Evidence synthesis: More intensive active surveillance frequency should be considered for patients with NCCN low-risk disease and a high GC score, given the higher probability of transitioning off active surveillance and subsequent progression. |
| NCCN Intermediate-Risk | ≤0.45 VS. ≥0.60 | RT vs. RT + ST-ADT |
Evidence: NRG/RTOG 0126 phase III randomized trial was profiled post-hoc with a prespecified analysis plan.3 The study demonstrated the independent prognostic effect of GC on biochemical failure, secondary therapy, DM, PCSM, MFS, and OS. Patients receiving RT alone with low GC scores had 10-year DM rates of 4%, compared with 16% for GC high risk. Evidence synthesis: RT alone should be considered for patients with a low GC score and NCCN intermediate-risk disease. The addition of ST-ADT should be considered for patients with a high GC score given their increased risk of DM and significant benefit of ST-ADT on DM, even with dose-escalated RT or brachytherapy boost. |
| NCCN High-Risk | ≤0.45 VS. ≥0.60 | RT + LT-ADT vs. RT + ST-ADT |
Evidence: A meta-analysis of three phase III randomized trials (NRG/RTOG 9202, 9413, and 9902) were profiled post-hoc with a prespecified analysis plan.4 The study demonstrated the independent prognostic effect of GC on biochemical failure, DM, MFS, PCSM, and OS. Patients with low GC scores had 10-year DM rates of 6%, compared with 26% for GC high risk. The absolute benefit of LT-ADT over ST-ADT was 11% for patients with high GC scores (NNT of 9), and 3% for patients with low GC scores (NNT of 33). Evidence synthesis: RT + LT-ADT should be recommended for most patients with NCCN high- risk disease regardless of the GC score outside of a clinical trial, even with dose-escalated RT or brachytherapy boost. However, patients with a GC low risk score should be counseled that the absolute benefit of LT-ADT over ST-ADT is smaller than for patients with GC high risk scores and when accounting for patient age, comorbidities, and patient preferences, it may be reasonable with shared decision-making to use a duration shorter than LT-ADT. |
| Post-RP BCR | <0.6 VS. ≥0.6a | RT vs. RT + ADT |
Evidence: Two phase III randomized trials post-RP were profiled post-hoc with prespecified analysis plans. NRG/RTOG 9601 demonstrated the independent prognostic effect of GC on DM, PCSM, and OS, and found that for patients with lower entry PSAs (<0.7 ng/mL), the 12- year DM rate benefit from hormone therapy for patients with GC lower risk vs. GC higher risk was 0.4% vs. 11.2%.5 The SAKK 09/10 phase III trial tested post-RP lower vs. higher dose RT alone. The study demonstrated the independent prognostic effect of GC on biochemical progression, clinical progression, secondary hormone therapy, DM, and MFS.6 Evidence synthesis: For patients with node-negative disease post-RP planned for early secondary RT (PSA ≤0.5 ng/mL) with GC low or intermediate risk, use of RT alone should be considered. For patients planned for early secondary RT with a GC high-risk tumor, use of secondary RT with ADT is recommended. Currently, it is unclear how best to use GC for patients receiving late post-RP secondary RT (PSA >0.5 ng/mL). Optimal ADT duration (ie, 6 vs. 24 months) based on GC score is unknown at this time. |
PROS-H, 4 of 8, 5 of 8
Table 3
GC = genomic classifier (Decipher), RP = radical prostatectomy, BCR = biochemical recurrence, RT = radiation therapy, ST = short-term, LT = long-term, ADT = androgen deprivation therapy, DM = distant metastasis, PCSM = prostate cancer-specific mortality, MFS = metastasis-free survival, OS = overall survival, NNT = number needed to treat, PSA = prostate specific antigen
- SAKK 09/10 combined GC low and intermediate risk due to relatively similar prognosis. NRG/RTOG 9601 dichotomized patients by GC low versus intermediate and high risk. However, due to the age of the tissue from NRG/RTOG 9601 (>20 years old) there is a known shifting of GC scores, and a more contemporary distribution of score distribution would approximate closer to combining GC low and intermediate risk together.
References
1. Simon, R. M. et al. J Natl Cancer Inst 101, 1446-1452, (2009). 2. Vince Jr, RA et al. Prostate Cancer Prostatic Dis 25, 677-683. 2022. 3. Spratt, DE et al. Int J Radiat Oncol Biol Phys 117, 370-377. 2023. 4. Nguyen, PL et al. Int J Radiat Oncol Biol Phys 116, 521-529. 2023.. 5. Feng, FY et al. JAMA Oncol 7(4), 544-552. 2021. 6. Dal Pra A et al., Ann Oncol 33(9), 950-958. 2022.
*Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.3.2024. © 2024 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN = National Comprehensive Cancer Network® (NCCN®).
Decipher Prostate is available in the United States as part of Veracyte’s CLIA-validated laboratory developed test (LDT) service, and FDA clearance is not required.
Decipher Prostate Helps Address Key Clinical Questions Across the Spectrum of Localized Prostate Cancer
Clinical Uses of Decipher After Biopsy
| Clinical Decision | Decipher Risk | May Consider |
|---|---|---|
| Active Surveillance Protocol | Low | Less Intense 1-5 |
| High | More Intense 1-5 |
| Active Surveillance OR Definitive Therapy | Low | Active Surveillance 1-5 |
| High | Definitive Therapy 1-5 |
| Radiation OR Radiation + ADT | Low | Radiation 1,4-6 |
| High | Radiation+ADT 1,4-7 |
| Duration of Hormone Therapy with Radiation | Low | Radiation + Short-Term ADT 7-8 |
| High | Radiation + Long-Term ADT 7-9 |
Clinical Uses of Decipher After Surgery
| Clinical Decision | Decipher Risk | May Consider |
|---|---|---|
| PSA Monitoring OR Treatment | Low | PSA Monitoring 9-11 |
| High | Treatment 9-11 |
| Radiation OR Radiation + ADT | Low | Radiation 12-14 |
| High | Radiation+ADT 12-14 |
Decipher Prostate was Developed to Predict Metastasis
- Metastasis is the best surrogate endpoint for overall survival for prostate cancer.
- Discovery and validation studies were completed in tumor tissue from a high-risk cohort of localized prostate cancer patients.
20% of these patients developed distant metastases within 5 years. - Differential analysis of metastatic versus non-metastatic tumor RNA expression identified robust differences in the expression
of the 22 genes that comprise the Decipher Prostate Genomic Classifier.

639 Post-RP Patients
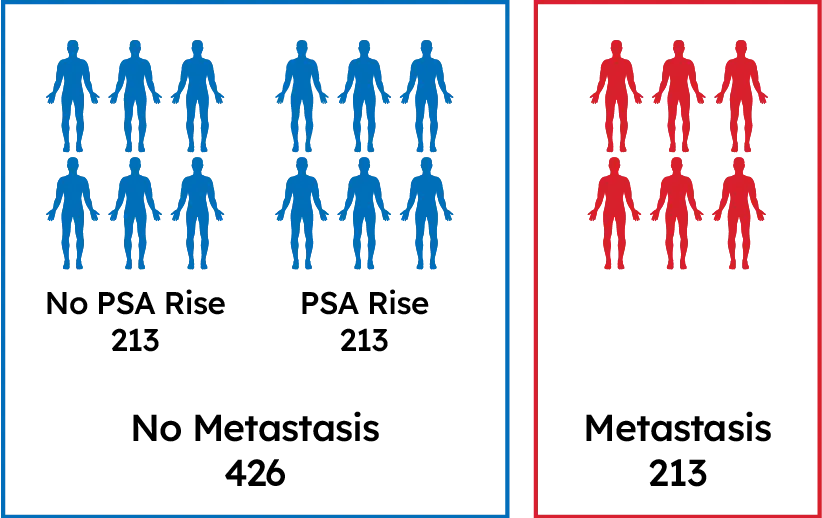
Patients with long term follow-up:
Gleason 8-10 or Pre-Op PSA>20 ng/mL or
Stage T3 or greater or SM+
Whole Transcriptome
Microarray
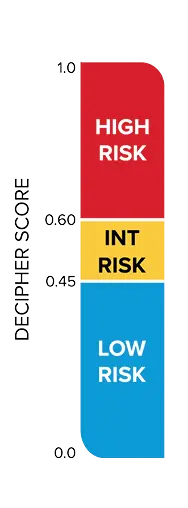
Decipher Risk
Well-annotated tumor registry with long-term treatment & outcomes data
Clinically high-risk patients with different 5-year outcomes: no PSA rise, PSA rise, & distant metastasis
Compared gene expression from tumor tissue of patients who developed metastasis vs. those who did not
Identified the 22 biomarkers that comprise the test

Decipher Prostate Resources
Decipher Prostate Requisition
Decipher Prostate Physician Brochure
Don’t see what you’re looking for?
Visit these pages for more information or leave us a message on the contact form.
Frequently Asked Questions
How can I place an order?
Physicians can place an order by filling out a requisition and submitting the required forms using fax, email, or the Decipher portal. Visit our How to Order page to learn more.
How can I set up a portal account to begin ordering?
Please fill out a contact form here and a representative will reach out to help set up a portal account.
How can I contact a local representative to learn more?
Please fill out a contact form here to contact a representative to learn more about Decipher testing.
What is included in a patient’s Decipher Prostate test report?
The first page of the test report includes the patient’s Decipher Score, Decipher risk group (low, intermediate, or high), interpretation language, and risk estimates of prostate cancer specific outcomes. The second page contains more detailed information relevant to the specific patient. Click here to view the sample test report.
Is the Decipher Prostate Genomic Classifier or Decipher Bladder Genomic Classifier FDA cleared?
The Decipher Prostate Genomic Classifier and the Decipher Bladder Genomic Classifier are available as part of Veracyte’s Clinical Laboratory Improvement Amendments (CLIA)-validated laboratory developed test (LDT) service. FDA approval or clearance is not required for the Decipher Prostate Genomic Classifier or Decipher Bladder Genomic Classifier.
Veracyte Labs SD, which performs Decipher testing is accredited/licensed by the following:
- College of American Pathologists
- Centers for Medicare and Medicaid Services (CMS) CLIA
- California Department of Public Health
- New York State Department of Health
- Pennsylvania Department of Health
- State of Rhode Island and Providence Plantations Department of Health Center for Health Facilities Regulation
- Maryland Department of Health, Office of Health Care Quality
Can the Decipher Prostate Genomic Classifier or Decipher Bladder Genomic Classifier be ordered if I live outside the United States?
Currently, Decipher testing is only available within the United States. Decipher testing is available as part of Veracyte’s CLIA-validated laboratory developed test (LDT) service. Veracyte’s exclusive global access to the nCounter Analysis System for diagnostic testing will allow us to make our genomic classifiers – currently performed in our centralized labs for patients in the U.S. – available to physicians and their patients globally. We plan to adapt Decipher genomic testing to the nCounter Analysis System in the next few years. Feel free to fill out our contact us form should you be interested in Decipher testing outside the United States.
References
- Vince Jr, RA et al. Prostate Cancer Prostatic Dis (2021).
- Kim, HL et al. Prostate Cancer Prostatic Dis 22, 399-405 (2019).
- Herlemann, A et al. Prostate Cancer Prostatic Dis 23, 136-143 (2020).
- Berlin, A et al. Int J Radiat Oncol Biol Phys 103, 84-91 (2019).
- Spratt, DE et al. J Clin Oncol 36, 581-590 (2018).
- Spratt, D. E. et al. J Clin Oncol 40, 269-269 (2022).
- Nguyen, PL et al. Prostate Cancer Prostatic Dis 20, 186-192 (2017).
- Nguyen, PL et al. Int J Radiat Oncol BIol Phys (2023).
- Den, RB et al. J Clin Oncol 33, 944-951 (2015).
- Ross, AE et al. Prostate Cancer Prostatic Dis 19, 277-282 (2016).
- Marascio, J et al. Prostate Cancer Prostatic Dis (2019).
- Feng, FY et al. JAMA Oncol 7(4): 544-552 (2021).
- Spratt, DE et al. Eur Urol 74, 107-114 (2018).
- Dal Pra A et al., Ann Oncol, 33(9); 950-958 (2022).
This page is intended for US healthcare professionals only.