The Decipher Prostate RP Genomic Classifier
test report overview can help you understand
components of the test report.

Page 1 contains the Decipher Score, risk estimates, and an interpretation of the score.

1. Decipher Score
- Determined by tumor biology alone, not influenced by clinicopathologic variables (e.g., Gleason, PSA)
- 22 genes, 7 biological pathways
- Continuous genomic risk score
- 3 categories of risk (low, intermediate, or high)
2. Risk Estimates
- Prostate cancer risk estimates were determined by numerical integration of >30,000 prostate cancer patients with available Decipher scores calibrated to >25,000 patients with long-term follow-up and from published meta-analyses.
- Risk of 5- & 10-year metastasis and 15-year prostate cancer mortality after radical prostatectomy.
3. Interpretation
Summary based on the patient’s Decipher score & relevant clinical findings from published studies including Decipher.
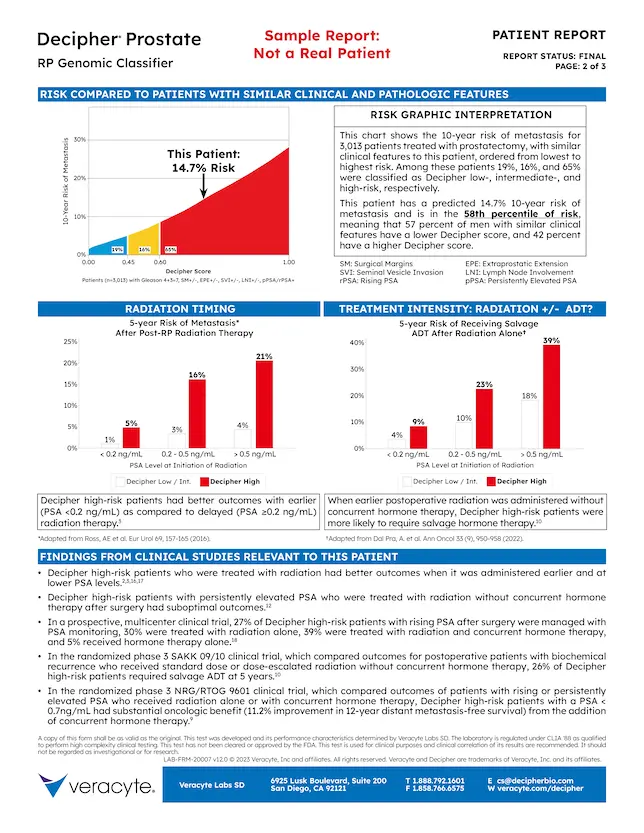
Page 2 contains case analysis, interpretation, and clinical findings.
4. Dynamic Report
The report is tailored to the clinical presentation of the patient
5. Risk Comparisons
- This patient’s 10-year risk of metastasis is compared to 987 patients with similar clinical features
- For these patients, Decipher risk groups are distributed as:
- 35% Low
- 16% Intermediate
- 49% High
- Distribution of risk is dependent on clinical risk group
6. Interpretation
- Explains risk comparison graph
- Specifies the percentile of risk for this patient
7. Treatment Graphics
- Evidence from Decipher Prostate RP studies
- Radiotherapy Timing (Left)
- Risk of metastasis with post-RP radiotherapy is dependent on the Decipher score and PSA level at time of treatment
- Treatment Intensity (Right)
- Risk of progression to ADT after radiotherapy alone in conjunction with the Decipher score and PSA level can help with the hormone therapy decision
8. Clinical Findings
Clinical study results relevant for the patient
Page 3 contains a test description, intended use info, confidence intervals, and references.

9. Test Description
- How the test is performed
- Definitions for each of the endpoints reported
- Provides detail on the cut-points for Decipher risk groups
10. Intended Use
Decipher Prostate RP is intended for use in men with localized prostate cancer after surgery with undetectable, persistent, or rising PSA
11. Confidence Intervals
The 95% confidence interval for each endpoint reported
12. References
For each of the clinical studies cited in the report